BOUTIQUEResouces
Based on research needs for delivering different types of nucleic acids/proteins, researchers often encounter the following three types of problems during cell transfection:
· Cells are relatively sensitive to chemical reagents, leading to decreased cell viability or cell death after transfection;
· A large variety of cell types are commonly used in research, and transfection efficiency varies significantly among different cell types. As a result, a single transfection reagent or experimental protocol cannot meet the experimental requirements;
· Delivering multiple types of nucleic acids or proteins requires selecting corresponding transfection reagents and conducting extensive experimental optimization to achieve high transfection efficiency.
Is there a chemical transfection reagent that can handle both conventional and hard-to-transfect cell types, has low cytotoxicity, and is suitable for delivering multiple types of nucleic acids/proteins? The TransIT-X2® Transfection Reagent, which combines multiple functions and high performance, can handle both common and hard-to-transfect cell types simultaneously. It eliminates the need for condition exploration and extensive repetitive experiments, allowing you to easily obtain your desired experimental results.
· It has been verified to exhibit excellent transfection efficiency, low cytotoxicity (especially compared to Lipofectamine® series reagents that form liposome complexes), and high performance in most cell types (such as adherent/suspension cells, primary cells, and neural cells);
· It is suitable for a wide range of research fields, including but not limited to stem cell research, CRISPR-based gene editing research, RNAi gene interference/silencing, stable cell line generation, low-toxicity transfection experiments, and co-transfection experiments;
· It enables efficient and stable simultaneous delivery of multiple types of nucleic acids/proteins, including but not limited to plasmid DNA, siRNA/miRNA, and CRISPR/Cas9 components.
Why does the Mirus TransIT-X2® Dynamic Delivery System possess such high performance and broad adaptability? This is attributed to Mirus’ powerful and efficient patented delivery platform design, which enables screening and optimization of transfection reagent components for multiple and multifunctional combinations. Founded in 1995, Mirus has focused on and specialized in the field of nucleic acid delivery for many years (Figure 1). From the earliest low-toxicity TransIT®-LT1 to the GMP-grade TransIT-VirusGEN® (which can be used for AAV and LV production), all products are designed and developed based on this delivery platform. To date, more than 10,000 research papers have been published using Mirus products, and these papers and Mirus’ database cover over 1,200 cell types. For more papers and data queries, please click here.
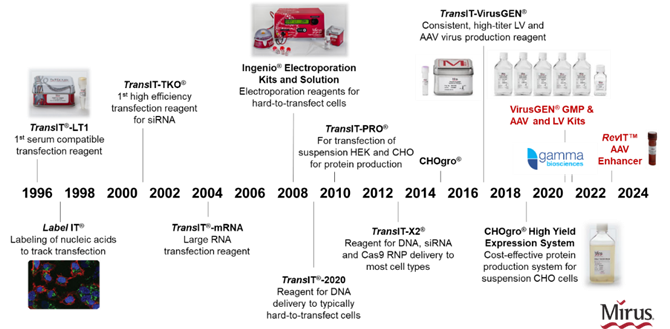
Figure 1 Mirus Product Milestones
Unlike traditional single-component-based transfection reagents (such as cationic polymers or cationic liposomes), Mirus combines its patented polymer and lipid technologies in multiple ways to further improve transfection efficiency for addressing different cell types or specific applications. Without forming liposomal complexes (i.e., liposomes, which usually have cytotoxicity), this multi-combination approach yields a variety of high-performance transfection solutions. These solutions overcome multiple obstacles in the cell delivery process, enabling more efficient transfection and lower cytotoxicity (Figure 2).
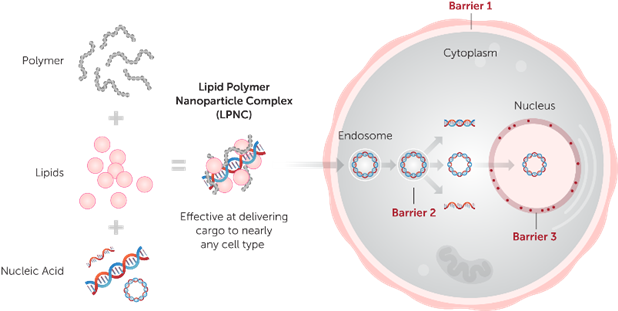
Figure 2 Schematic Diagram of the Principle of Mirus Transfection Reagents
Based on the design of Mirus' powerful and efficient patented delivery platform mentioned above, combined with its unique intelligent iterative design process— which involves multi-component, multi-combination screening, optimization, and subsequent validation—each component in the transfection technology is optimized (Figure 3). Mirus has launched a series of classic transfection reagents to meet diverse needs:
1. Broad-Spectrum, High-Performance, Low-Toxicity Transfection Reagent Family:
TransIT-X2®, TransIT®-LT1, TransIT®-2020
2. Transfection Reagent Family for Specific Cell Types:
TransIT®-293, TransIT®-BrCa, TransIT®-CHO, TransIT-HeLaMONSTER®, TransIT®-Insect, TransIT®-Jurkat, TransIT®-Keratinocyte
3. Transfection Reagent Family for Specific Applications:
· Viral Production: VirusGEN® Transfection Reagents and Matching Kits, TransIT®-Lenti
· Protein/Antibody Production: TransIT-PRO®, CHOgro® Expression System, CHOgro® High Yield Expression System
4. Transfection Reagent Family for Oligonucleotide Delivery:
TransIT®-Oligo, TransIT-siQUEST®, TransIT-TKO®
5. mRNA and Long-Chain RNA Delivery:
TransIT®-mRNA
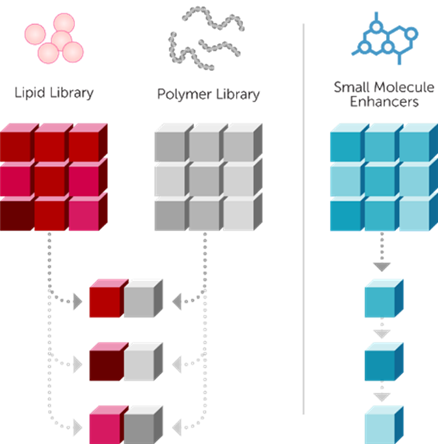
Figure 3 Schematic Diagram of Mirus' Unique Intelligent Iterative Design for Transfection Reagent Optimization Process
Performance Data Presentation of Mirus TransIT-X2® Transfection Reagent
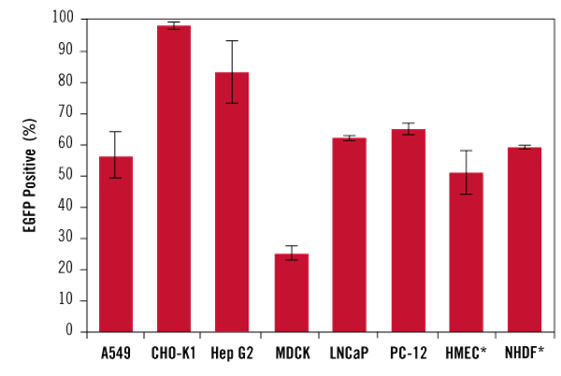
Figure 4 TransIT-X2® Exhibits High Transfection Efficiency in Multiple Cell Types, Including Primary Cells
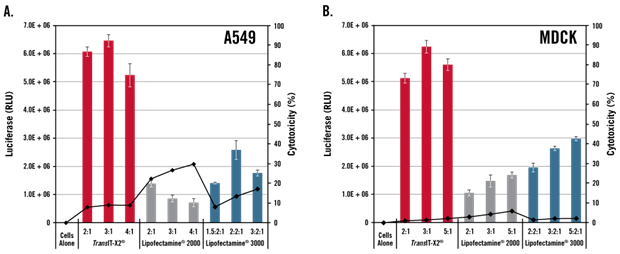
Luciferase-encoding plasmid DNA was transfected into A549 (A) or MDCK (B) cells using TransIT-X2® (Mirus Bio), Lipofectamine®2000 (Thermo Fisher Scientific), or Lipofectamine®3000 (Thermo Fisher Scientific). At 24 hours post-transfection, transfection efficiency was evaluated by luciferase activity, and cytotoxicity was assessed by quantifying LDH released from damaged cells. Compared with cells treated with Lipofectamine®2000 or Lipofectamine®3000, TransIT-X2® exhibited higher fluorescence intensity and lower cytotoxicity at the optimal reagent:DNA ratio.
Figure 5 Compared with Lipofectamine® series transfection reagents (which are single-component and form cationic liposomal complexes), TransIT-X2® shows higher transfection efficiency and lower cytotoxicity
Experimental Tips: For more specific cell types and recommendations on reagent usage, please refer to the details in:Cell-type specific transfection protocol recommendations (PDF).
Performance Data Presentation of Mirus TransIT-X2® in RNAi Experiments
Functional research related to gene silencing plays a crucial role in molecular and cellular biology, and chemical transfection also plays an important part in this research field. Common natural RNA molecules involved in the RNAi pathway include:
· Small interfering RNAs (siRNAs): Short double-stranded RNAs (20-25 bp) generated by the cleavage of double-stranded RNA (dsRNA);
· MicroRNAs (miRNAs): A class of short, single-stranded RNAs (20-22 nt) produced after the processing of non-coding RNAs.
Another method for inhibiting gene expression using RNAi is short hairpin RNA (shRNA). These short RNA sequences can be expressed via viral or non-viral vector methods. For more detailed information, please refer to Mirus Applications | RNAi Gene Silencing。
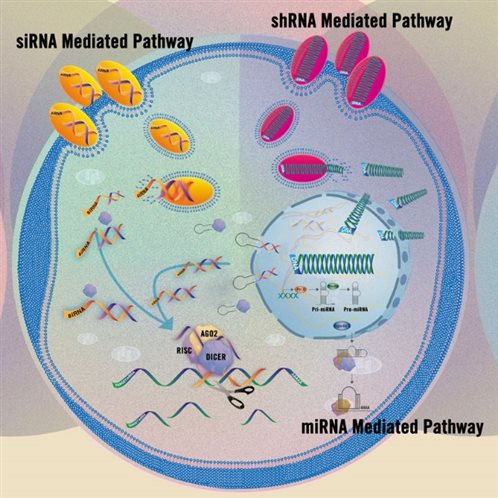
Figure 6 Schematic Diagram of Different RNAi Pathways
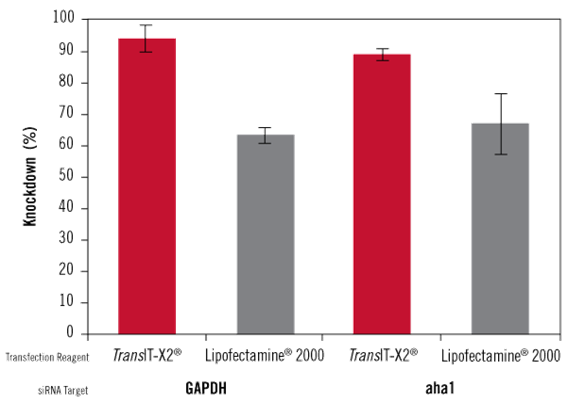
Figure 7 In gene silencing studies based on siRNA nucleic acid delivery, TransIT-X2® exhibits higher gene silencing efficiency compared to Lipofectamine®2000
The TransIT-X2® Dynamic Delivery System and Lipofectamine®2000 transfection reagents were used to transfect siRNAs (targeting endogenous proteins—GAPDH and AHA1). For the control group, normal human dermal fibroblasts (NHDF) were used to deliver non-targeting siRNA. The amount of TransIT-X2® added was 4 μl, the amount of Lipofectamine®2000 added was 6 μl, and the amount of siRNA added was 25 nM for both reagents.
qRT-PCR was performed to measure the mRNA levels of GAPDH or AHA1 relative to 18s rRNA. At 48 hours post-transfection, the mRNA levels were normalized to those of the non-targeting control group. The error represents the standard deviation from three replicate well experiments.
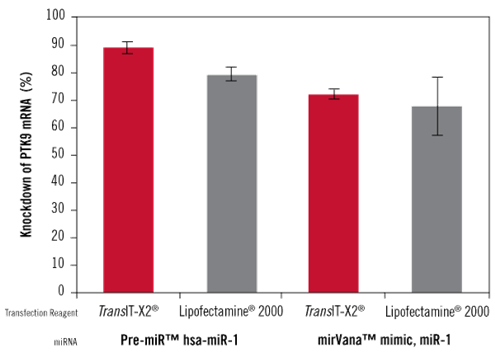
Figure 8 In gene silencing studies based on miRNAs (miRNA Precursor and miRNA mimic), TransIT-X2® exhibits higher gene silencing efficiency compared to Lipofectamine®2000
The TransIT-X2® Dynamic Delivery System and Lipofectamine®2000 transfection reagents were used to transfect Pre-miR? hsa-miR-1 miRNA Precursor or mirVana? miRNA mimic, miR-1 (both have been validated to reduce PTK9 mRNA expression levels). Pre-miR? negative control was used to assess the baseline level of mRNA expression.
The amount of both TransIT-X2® and Lipofectamine®2000 reagents added was 3 μl, with 50 nM miRNA added. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to measure PTK9 mRNA expression levels relative to 18s rRNA, and the results were normalized against the negative control.
Performance Data of Mirus TransIT-X2® in CRISPR Gene Editing Experiments
The modified and optimized bacterial CRISPR/Cas9 system has been widely applied in gene editing of mammalian cells. There are two common key components in CRISPR-based gene editing experiments: Cas9 protein and guide RNA (gRNA). When Cas9 protein cleaves the target genomic DNA, it triggers two endogenous repair mechanisms, namely non-homologous end joining (NHEJ) and homology-directed repair (HDR), to address DNA breaks formed in cells.
The NHEJ-based cellular repair pathway is an imprecise and error-prone repair mechanism, which can introduce indels (insertions or deletions) that lead to loss of gene function. In contrast, the HDR-based cellular repair pathway requires additional homologous DNA as a repair template to achieve "precision" repair, allowing the insertion of specific genes or long-fragment sequences into the target gene.
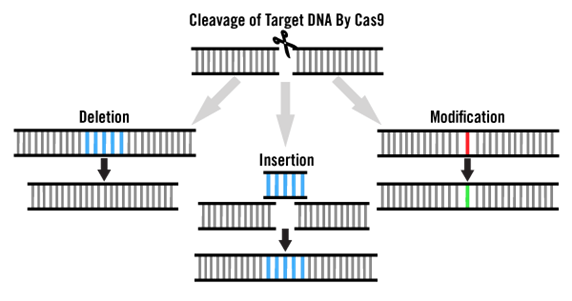
According to researchers' different experimental needs, CRISPR gene editing can be set up in multiple experimental configurations. This means addressing the transfection or even co-transfection of different nucleic acid types, with examples as follows:
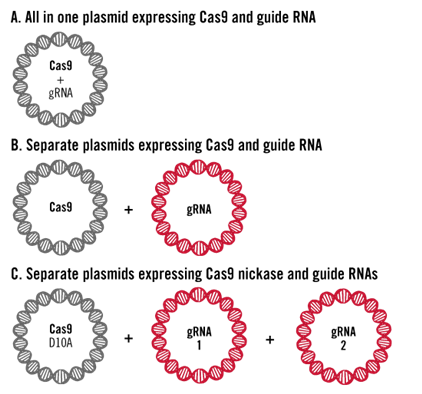
1. Plasmid pDNA Transfection
The two key components of a CRISPR experiment—Cas9 protein and gRNA—can be designed to be co-expressed in a single plasmid DNA (A); alternatively, a two-plasmid system can be used, where one plasmid expresses Cas9 protein and the other expresses gRNA (B); when a Cas9 nickase is used, two gRNAs are required, which may necessitate a three-plasmid system for delivery experiments.
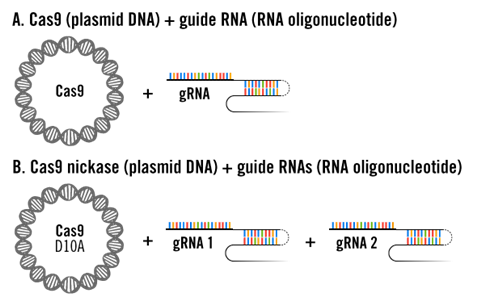
2. Co-transfection of Plasmid DNA and RNA Oligonucleotides
In this case, different from the above experimental design, gRNA is delivered in the form of oligonucleotides, which means the delivery experiment requires simultaneous transfection of plasmid DNA and RNA oligonucleotides (A and B).
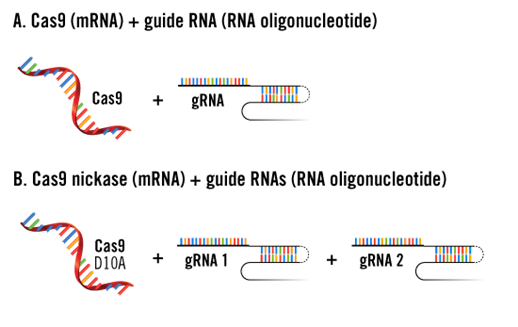
3. Co-transfection of mRNA and RNA Oligonucleotides
To avoid off-target effects caused by genomic integration of plasmid DNA, mRNA encoding Cas9 protein and gRNA (RNA oligonucleotides) can be co-transfected.
3. Delivery of Cas9/gRNA RNP Complexes
As the commercialization of Cas9 protein and gRNA becomes increasingly mature, more and more researchers choose to directly purchase high-fidelity Cas9 protein and gRNA oligonucleotides with additional chemical modifications (which enhance their stability in cells) from third-party suppliers. Apart from greatly shortening and simplifying the CRISPR experimental workflow, the direct delivery of RNP complexes offers higher specificity and editing efficiency compared to the approaches that rely on plasmid- or mRNA-based synthesis of Cas9 and gRNA.
Experimental Tips:
For the specific experimental instructions optimized for the TransIT-X2® Dynamic Delivery System (tailored to the different CRISPR experimental designs and delivery scenarios mentioned above), the download link is as follows:
TransIT-X2® for CRISPR Plasmid + gRNA Delivery (PDF)
TransIT-X2® for CRISPR Plasmid Delivery (PDF)
TransIT-X2® for CRISPR RNP + ssODN Delivery (PDF)
TransIT-X2® for CRISPR RNP Delivery (PDF)
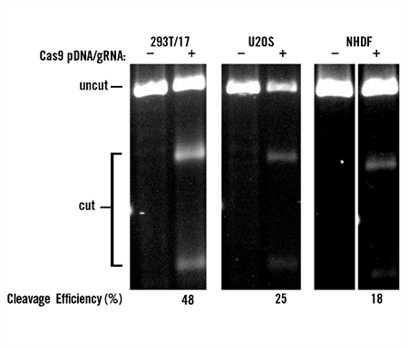
The TransIT-X2® Dynamic Delivery System (2 μl/well in 24-well plates, Mirus Bio) was used to co-transfect 0.5 μg of plasmid DNA (expressing Cas9 protein) and 50 nM of 2-part gRNA (targeting the PPIB gene) into HEK293T/17, U2OS, and NHDF cells. At 48 hours post-transfection, T7E1 assay was performed to verify the cleavage efficiency.
Figure 9 Co-transfection of Plasmid DNA and gRNA Oligonucleotides: TransIT-X2® achieved high gene editing efficiency (18-48%) when co-transfecting Cas9 plasmid DNA and gRNA (RNA oligonucleotides) into 293T/17, U2OS, and NHDF cells, respectively
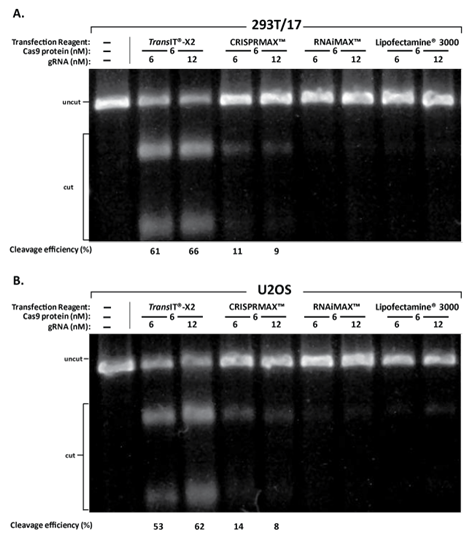
The 2-part gRNA (targeting PPIB gene, IDT) and Cas9 protein (PNA Bio) formed RNP complexes, which were respectively delivered into 293T/17 and U2OS cells using TransIT-X2® Dynamic Delivery System (1 μl/well, Mirus Bio), Lipofectamine® CRISPRMAX? (1.5 μl/well, ThermoFisher), Lipofectamine® RNAiMAX (1.5 μl/well, ThermoFisher), and Lipofectamine® 3000 (1.5 μl/well, ThermoFisher). Two usage amounts of gRNA were tested (6 nM or 12 nM) with the same amount of Cas9 protein added (6 nM). At 48 hours post-transfection, the T7E1 assay was performed to verify the cleavage efficiency.
Figure 10 Delivery of Cas9/gRNA RNP Complexes: TransIT-X2® exhibits higher cleavage efficiency compared to Lipofectamine® series transfection reagents
Advantages of Mirus TransIT-X2® Product
? A new broad-spectrum transfection reagent developed based on multiple components (suitable for various cells, including hard-to-transfect cells);
? Capable of co-transfecting nucleic acids and proteins, including but not limited to DNA, siRNA/miRNA, and CRISPR/Cas9 complexes;
? Does not form liposomal complexes, reducing cytotoxicity;
? Meets multiple applications: gene overexpression, gene silencing, gene editing, stem cell research, stable cell line construction, etc.
Related Product Information
|
Product Name |
Specification |
Item Number |
|
TransIT-X2® Dynamic Delivery System |
1 × 0.3 ml |
MIR 6003 |
|
1 × 0.75 ml |
MIR 6004 |
|
|
1 × 1.5 ml |
MIR 6000 |
|
|
5 × 1.5 ml |
MIR 6005 |
|
|
10 × 1.5 ml |
MIR 6006 |
End-of-Article Benefit:
Follow Ximeijie's official WeChat public account "xmjsci", reply with the keyword "X2 Trial", and fill in the information to have the opportunity to receive a free 100μl trial sample of TransIT-X2® Transfection Reagent. After a successful trial, you can also enjoy a discount when purchasing the full-size TransIT-X2®! Come and apply for the trial and make your purchase now!
![]()
Founded in 1995, Mirus (USA) is one of the earliest companies in the world to develop high-efficiency and low-toxicity transfection reagents, and also one of the leading suppliers of such reagents currently. Recognized as a pioneer in transfection reagent development, Mirus has achieved numerous outstanding results that have gained global recognition and high praise from a wide range of users.
Beijing XMJ Technology Co., Ltd. is the authorized distributor of Mirus in China. Adhering to a professional and rigorous attitude, we have been committed to providing customers with high-quality products and services. If you are interested in the aforementioned products, please feel free to send email to info@xmjsci.com or visit the official website www.gq44.cn to obtain more product information.


.png) 京公網(wǎng)安備 11010802028692號(hào)
京公網(wǎng)安備 11010802028692號(hào)