BOUTIQUEResouces
With the continuous in-depth research and development of viral vector technology, it has provided an ideal tool for genetic material delivery and become the main delivery technology in current cell therapy and gene therapy. However, researchers are facing significant challenges in the purification process of cell and gene therapy products. Most of the current purification processes are derived from solutions developed for monoclonal antibodies and are not suitable for the purification of viral vectors. Moreover, traditional resin-based chromatography has obvious limitations, such as low recovery rate, long time consumption and high cost.
AstreAdept? is a new type of nanofiber material that can effectively address the problems of long time consumption, low recovery rate and high impurity content in the purification of large-size and fragile viral particles. Here, we demonstrate the integration of this technology into the Nereus LentiHERO? in centrifuge tube format, bringing its advantages to laboratory-scale viral vector purification.
High Transient Binding Surface Area for More Efficient Lentivirus Vector Processing
AstreAdept? adopts two different electrospun materials (cellulose: 575 nm (+/- 50 nm) and non-cellulose: 200 nm (+/- 50 nm)) interwoven to form a composite membrane structure with excellent properties. This structure allows the product solution to quickly diffuse to a high binding surface area under high flow rate conditions (Figure 1), enabling unobstructed access to the pores. LentiHERO?, which integrates this technology into the chromatographic purification matrix, ushers lentivirus purification into a new era.
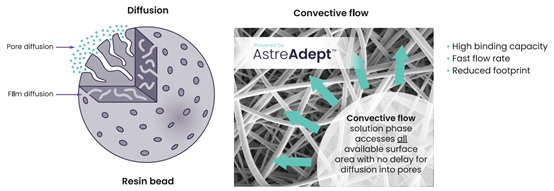
Figure 1. AstreAdept? Novel Composite Nanofiber Material Addresses Bottlenecks in the Purification of Fragile Macromolecules
Advantages of Unobstructed Liquid Flow System:
· Larger binding surface area
· High recovery rate
· High flow rate
Nereus LentiHERO? for Lentivirus Purification
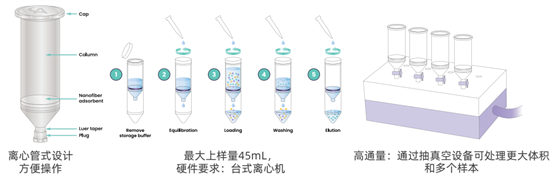
Figure 2. Nereus LentiHERO? Enables Rapid Lentivirus Purification in Only 5 Centrifugation Steps
Nereus LentiHERO? Significantly Improves Lentivirus Recovery Rate
With high dynamic binding capacity and high recovery rate, it enhances the efficiency of laboratory-scale lentivirus production.
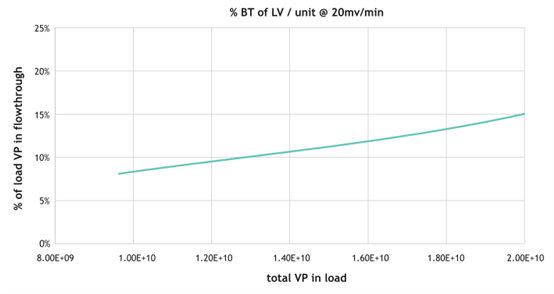
Figure 3. At 15% breakthrough, the dynamic binding capacity per spin column is 1.9E+10. The Nereus LentiHERO? spin column was loaded at a flow rate of 20 mV/min, and the flow-through was collected in 10 mL of liquid. Using p24 ELISA for analysis, the percentage of VP breakthrough was calculated based on the total VP loaded.
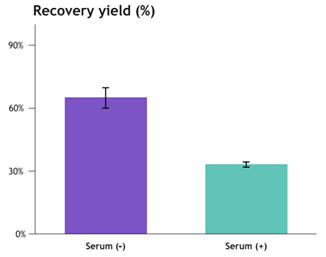
Figure 4. Lentivirus Recovery Rate in Serum-Free vs. Serum-Containing Culture Systems: Percentage of Lentivirus in Purified Samples Relative to Viral Stock Solution
Serum (-) n=3, Serum (+) n=2
· Dynamic Binding Capacity Remains Unaffected Under High Flow Rate Conditions
· Retention time < 1s
· LV recovery rate > 60% in serum-free culture system
Nereus LentiHERO? Reduces Host Cell Protein (HCP) Residues by Over 95%
Reducing HCP residues is equally important in laboratory-scale sample production, as it can significantly minimize unnecessary immune responses.
Samples Purified with Nereus LentiHERO?
· 95% removal of host proteins
· No host proteins detected in the eluate via SDS-PAGE
· Significant removal of host proteins regardless of the presence or absence of serum in the culture system
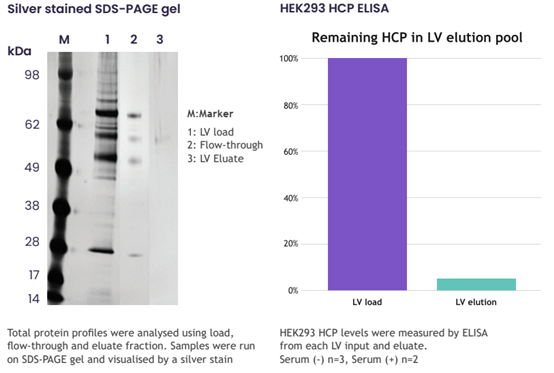
Figure 5. Nereus LentiHERO? Can Remove Over 95% of Host Proteins
Nereus LentiHERO? Has Minimal Impact on Viral Structure and Infectious Potency
Lentiviral particles purified by Nereus LentiHERO? retain their original function, morphology, and size:
· Key structural proteins remain intact, such as the Gag protein that encodes the capsid, matrix, and nucleocapsid.
· The size and morphology of lentiviral particles show no changes.
· The infectious activity of purified lentiviruses is not reduced.
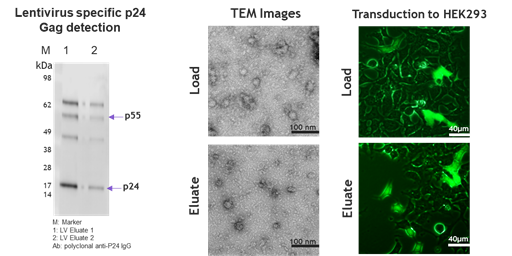
Figure 6. Through Western Blotting (WB), electron microscopy, and cell infection assays, comparisons were made between lentiviruses before and after purification by Nereus LentiHERO?. The results showed that the integrity of lentiviral structural proteins, particle shape and size, and infectious activity were not significantly affected.
Nereus LentiHERO? Combines Yield, Purity, and Time Efficiency
· High-throughput viral purification can be achieved with just a benchtop centrifuge.
· While removing host cell proteins, the sample elution volume is reduced (concentration effect).
· Further concentration of high-purity, small-volume samples saves more time.
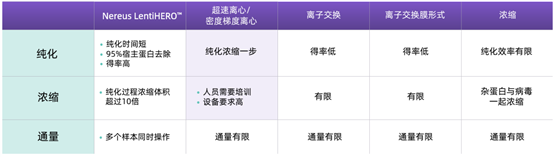
Performance Summary of Nereus LentiHERO?
· A novel AstreAdept? technology based on nanofiber membranes empowers the purification process of viral vectors.
· The easy-to-operate design enables rapid purification and high recovery rate of functional lentiviral particles.
· It can significantly reduce the content of impurities such as host cell proteins (HCPs).
· It can process multiple samples simultaneously, breaking through the bottleneck of sample preparation in viral vector development.
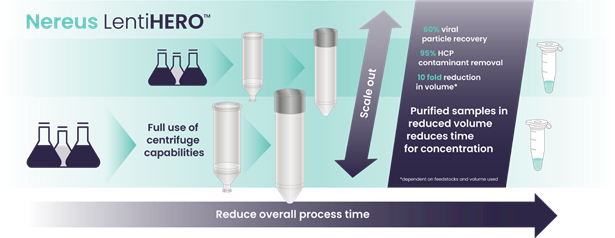
Figure 7. Lentivirus (LV) Purification with High Yield, High Throughput, and High Purity, Suitable for Small-Volume Lentiviral Samples and Short Operation Time
In summary, this technology provides a more cost-effective and time-efficient solution, enhances researchers' development of new cell and gene therapy projects, and facilitates the accessibility of novel biotherapeutic drugs to a large number of patients.
Product Information*:
|
Product Name |
Item Number |
Specification |
|
Nereus LentiHEROTM |
NL100100 |
2 unit pack |
|
|
|
|
*The LentiHERO® 1 and LentiHERO® 10 pre-packed column products, which adopt the same technology and are suitable for process scale-up, are now available. For inquiries, please contact Beijing Ximeijie Technology Co., Ltd., the agent of Astrea Bioseparations.

Astrea Bioseparations was incubated at the University of Cambridge in 1987. With over 30 years of experience in the research, development and production of chromatographic media, it is a world-class supplier of chromatographic media products and services. To date, more than 21 manufacturing processes using Astrea's products have obtained approvals from the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency).
In Greek, "Astrea" means "star maiden", which symbolizes justice, innocence, purity and precision. The use of the name "Astrea Bioseparations" reflects our consistent focus on purity, precision and humanity in our work.
Astrea Bioseparations has 3 R&D and production bases around the world, dedicated to providing industry-leading chromatographic media and technical services for the fields of biomacromolecules and CGT (Cell and Gene Therapy). The novel nanofiber-based chromatographic technology launched by Astrea Bioseparations addresses the problems of low capacity and time-consuming processes of traditional bioseparation tools. It enables faster, more environmentally friendly and more cost-effective purification processes, which fully meets the needs of today's biotherapeutic innovation.

Beijing XMJ Technology Co., Ltd. is the first-level agent of the Astrea Bioseparations brand in China. If you are interested in the above-mentioned products, please feel free to send email to info@xmjsci.com or log on to XMJ's official website www.gq44.cn for inquiries and orders.


.png) 京公網(wǎng)安備 11010802028692號(hào)
京公網(wǎng)安備 11010802028692號(hào)