BOUTIQUEResouces
For biological products manufactured using human/animal-derived cells, tissues, body fluids, and other materials as initial production raw materials, human or animal-derived raw materials or excipients may be added during the preparation process or in the final formulations. These initial raw materials, raw materials, and excipients used all carry potential risks of viral contamination. To reduce the risk of viral contamination in biological products, relevant regulations from domestic and international regulatory authorities require that the production process of biological products includes manufacturing steps with the ability to inactivate/remove viruses, so as to ensure the biosafety of the products.
Currently, most biopharmaceutical companies generally choose professional viral clearance validation service providers to conduct viral clearance validation, such as Institutes for the Control of Pharmaceutical and Biological Products, CROs/CMOs, third-party testing companies engaged in biomedical fields, or relevant filter manufacturers. On one hand, conducting viral clearance validation business requires virus laboratories with strict biosafety levels, which must apply for P2 (Biosafety Level 2) filing in accordance with the pathogenic microorganism protection standards. On the other hand, carrying out this business also involves the procurement of scaled-down model instruments and equipment, training of professional personnel, and daily operation of laboratories. It covers the entire process from cell culture and bank establishment, virus preparation and purification to process testing and validation, which consumes considerable human, material, and financial resources and imposes additional economic burdens on biopharmaceutical companies.
To alleviate this problem, Cygnus MockV? Solution offers cutting-edge technologies and solutions, providing two types of mock viral particles (MVM-MVP and RVLP) that simulate the physical and chemical properties of live viruses as much as possible. This enables biopharmaceutical researchers to easily conduct early process development and process characterization in conventional laboratories, predict the viral clearance effect of purification process steps, and accumulate data. Furthermore, it reduces reliance on viral clearance validation service providers during the development phase and accelerates the process of drugs reaching the market as soon as possible (Figure 1).
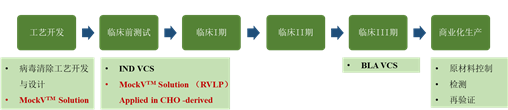
Figure 1. Application Points of Cygnus MockV in the Viral Clearance Validation Timeline
Introduction to the MockV? Product Series
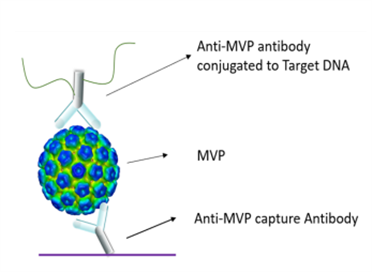
Figure 2. Detection Principle and Kit
Advantages and Features:
1. No need for complex live virus preparation and purification processes, especially for X-MuLV;
2. Fast detection speed, requiring only 4-5 hours;
3. Can be completed under conventional laboratory conditions;
4. Each kit contains reagents for 10 tests, enabling multiple tests on different processes;
|
Product Name |
Item Number |
Specification |
|
MockVTM MVM Kit |
M219 |
1kit (10 tests) |
|
MockVTM RVLP Kit |
M230 |
1kit (10 tests) |
Case Study #1 - Application of MVM MVP in AAV Purification Process
Conclusion: MVM-MVP provides an accurate and cost-effective method for predicting viral clearance of chromatographic separation technology in AAV production processes.
Reference:(Published)-Viral clearance in a Downstream AAV Process, Case Study Using a Model Virus Panel and a Noninfectious Surrogate
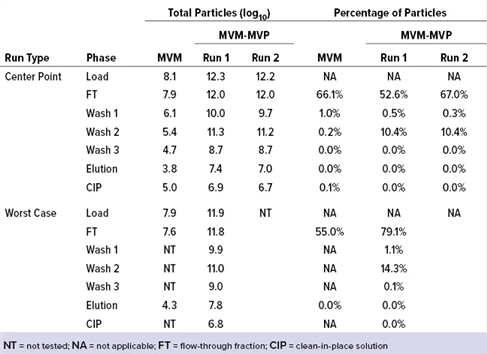
Table1.Affinity Resin Data From Experiments with MVM and MVM-MVPs
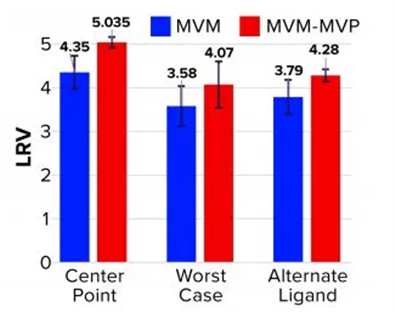
Table2. LRV Determinations for Affinity Resin Run Spiked with MVM and MVM-MVPs
Case Study #2 - Application of RVLP in Viral Clearance Evaluation of Biologics Expressed in CHO Cells
Conclusion: The latest updates to ICH Q5A (R2) make it possible to apply RVLP during viral clearance validation in the future.
Reference(Published)-Using Retrovirus like Particles for Downstream Viral Clearance Evaluation
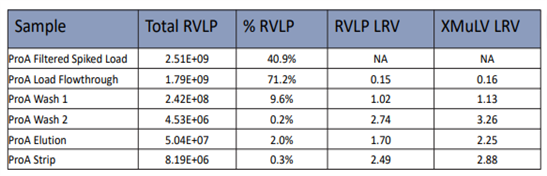
Table1. Protein A RVLP VS X-MuLV LRV Results
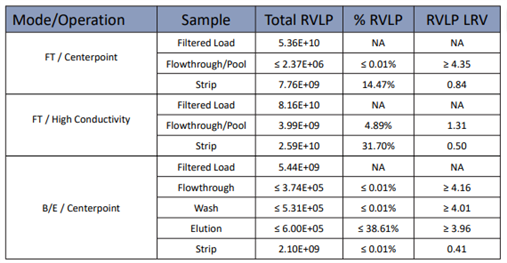
Table2. AEX RVLP LRV Results
Cygnus Technologies, LLC. provides reliable products and analytical methods for the biotechnology and biopharmaceutical industries, aiming to accelerate the R&D phase and improve product quality. The bioprocess residual kits developed and manufactured by Cygnus are used to detect specific impurities in more than 50 different expression systems. As an expert in high-sensitivity analytical technologies focused on immunoassays for biotechnological applications, Cygnus’ products and services have been used by nearly all major biopharmaceutical companies for over 25 years.
Beijing XMJ Technology Co., Ltd., as the exclusive distributor of Cygnus in China, has established long-term and stable cooperative relationships with many well-known domestic pharmaceutical companies and CRO/CMO enterprises. Over the years, XMJ’s products and services have helped many enterprises accelerate the R&D phase, improve drug quality, purity and safety, speed up the optimization of R&D processes, shorten product time-to-market, and reduce QC costs. If you are interested in the above-mentioned products, please feel free to send email to info@xmjsci.com or visit the website www.gq44.cn for more information.


.png) 京公網(wǎng)安備 11010802028692號(hào)
京公網(wǎng)安備 11010802028692號(hào)