BOUTIQUEResouces
During the manufacturing process of biological products, host cell DNA (HCD) may remain as a residue. This could lead to oncogenes or other high-risk genetic materials being present in the final pharmaceutical formulations. To mitigate this risk, regulatory authorities have established limits for allowable HCD residues. Depending on the cell line used and the administration protocol, the permissible range of HCD residues falls between 10 and 100 picograms (pg) per dose. Regarding the detection of HCD residues, the Chinese Pharmacopoeia 2025 General Chapter 3407 outlines three methods: DNA probe hybridization, fluorescent staining, and quantitative PCR (qPCR). In contrast, the United States Pharmacopeia (USP) General Chapter <509> "Residual DNA Testing" recommends probe-based DNA quantification as a validation method. This method is used to detect recombinant biological products manufactured in Escherichia coli (E. coli) or Chinese Hamster Ovary (CHO) cell lines, ensuring superior sensitivity and accuracy. Throughout the entire manufacturing process, quality control personnel are required to assess DNA levels. These samples may contain other impurities and high concentrations of active pharmaceutical ingredients (APIs), making a reliable and sensitive DNA quantification method essential.
The Chinese Pharmacopoeia 2025 Edition has added HCD detection requirements for multiple monoclonal antibody (mAb) drugs, including trastuzumab for injection, infliximab, adalimumab, bevacizumab, and rituximab. Additionally, certain vaccines—such as the inactivated Sabin-strain poliovirus vaccine (with a limit of <50 pg per dose)—explicitly require detection using the third method (quantitative PCR) specified in General Chapter 3407. Simultaneously, a new chapter, General Requirements for Antibody-Drug Conjugate (ADC) Products for Human Use, has been added. This chapter incorporates antibody-drug conjugates (ADCs) into regulatory oversight and clearly mandates monitoring of exogenous impurities.
The newly launched AccuRes? series of DNA quantification kits by Cygnus Technologies are specifically designed for detecting HCD residues in biological products recombinantly expressed in CHO, human, or E. coli cell lines. High-concentration samples can be tested with minimal dilution, thereby effectively lowering the limit of detection (LOD).
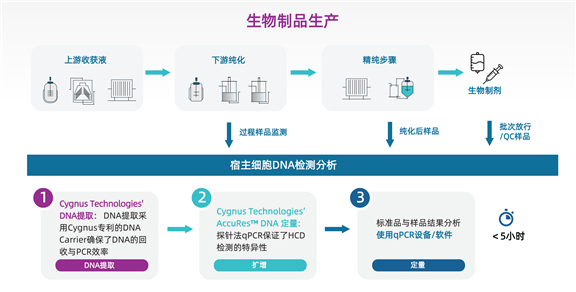
Advantages and Features of Cygnus AccuRes? Series HCD Detection Kits:
? Accuracy – Probe-based qPCR ensures specificity in HCD detection for target cell lines (e.g., CHO, HEK, E. coli), preventing false negatives of target HCD;
? Superior Precision – The system with CleanAmp® dNTPs and hot-start Taq DNA polymerase effectively reduces non-specific initiation during PCR amplification;
? Flexibility – Compatible with all qPCR instruments capable of detecting FAM fluorescent signals, reducing costs associated with additional equipment purchases;
? All-in-one kit supports the entire process from sample to qPCR – The kit includes DNA extraction reagents (tube method or plate method), AccuRes? PCR master mix, primer/probe master mix, and DNA standards;
? Sensitivity – Limit of Detection (LOD) is 0.6 fg/μl.
Operation Procedure of Cygnus AccuRes? Series HCD Detection Kits
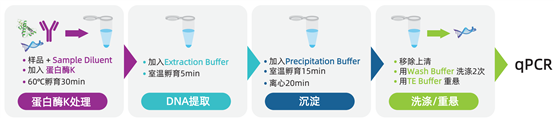
DNA Extraction
Cygnus' DNA extraction method utilizes a novel DNA carrier, which enables the recovery of HCD from trace-level residual DNA and facilitates DNA detection and quantification in an environment free from protein, salt, and detergent contamination. This improves the reproducibility and stability of DNA detection and amplification compared to other extraction methods.
Amplification
The Cygnus AccuRes? series kits employ a highly specific primer/probe system for amplifying target HCD. The nucleic acid probe labeled with FAM fluorescence is quenched by BHQ-1? in each PCR reaction cycle. The advanced reaction system consisting of CleanAmp® dNTPs and hot-start Taq DNA polymerase reduces non-specific amplification, further ensuring the specificity, sensitivity, and stability of qPCR amplification. Meanwhile, the preparation of the qPCR system can be performed at room temperature.
Quantification
The standard curve constructed using the Ct values of the kit's standards allows the detection results to be calculated as pg/10μL of residual host cell DNA. Furthermore, the HCD concentration can be converted into units such as ng/mL, ng/mg, or ng/dose. Based on this method, the minimum quantifiable concentration of residual DNA is 0.6 fg/μL.
Test Data of Cygnus AccuRes? Series HCD Detection Kits
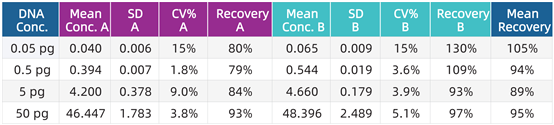
Accuracy and reproducibility tests: Two operators (A and B) performed duplicate detections on known DNA samples over two days, and the standard deviation (SD), coefficient of variation (CV), and recovery rate (%) of the detection results were calculated (see the table below).
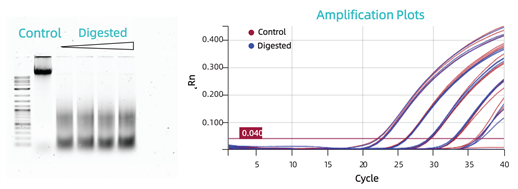
Comparison of Extraction and Amplification Effects between Intact and Degraded CHO DNA (at Different Degrees). Gel electrophoresis images of intact genomic DNA (Control) and DNA treated with AluI enzyme for 30 minutes, 1 hour, 1.5 hours, and 2 hours (Digested), respectively. The linear ranges of amplification for samples in the control group and the enzyme-treated group (30-minute treatment) are basically consistent (see the figure below).
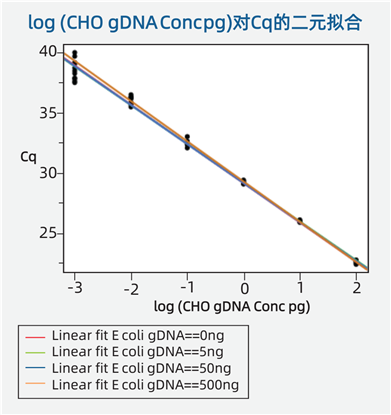
In the Presence of Exogenous DNA, CHO Primers/Probes Still Exhibit a Broad Linear Range and Specificity. E. coli DNA at different concentrations does not affect the standard curve of CHO DNA (see the figure below).
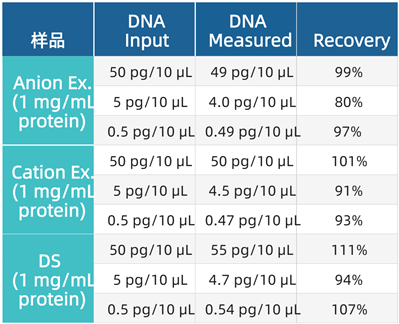
Comparison of Test Results Between In-Process Samples and Drug Substance (DS). The recovery rates of CHO DNA were compared across protein samples purified by anion exchange or cation exchange (at 1 mg/mL) and drug proteins (see the table below).
Ordering Information for Cygnus AccuRes? Series HCD Detection Kits
|
Product Name |
Item No. |
Specification |
|
CHO AccuRes? DNA Quantification Kit in Tubes |
D1555T |
1 kit |
|
CHO AccuRes? DNA Quantification Kit in Wells |
D1555W |
1 kit |
|
CHO AccuRes? Quantitative DNA Kit |
D1555 |
1 kit |
|
E. coli AccuRes? DNA Quantification Kit |
D1415 |
1 kit |
|
Human AccuRes? Quantitative DNA Kit |
D1165 |
1 kit |
Coming soon:
|
Product Name |
Item No. |
Specification |
|
Vero AccuRes? DNA Quantification Kit in Tubes |
D1975 |
1 kit |
|
NS/0 AccuRes? DNA Quantification Kit in Wells |
D1225 |
1 kit |
|
SF9 AccuRes? Quantitative DNA Kit |
D1845 |
1 kit |
|
P. pastoris AccuRes? Quantitative DNA Kit |
D1145 |
1 kit |

Cygnus Technologies, LLC. provides products and analytical methods for the biotechnology and biopharmaceutical industries, aiming to accelerate the R&D phase and improve product quality. Cygnus develops and manufactures bioprocess residue test kits, which are used to detect specific impurities from over 50 different expression systems. As an expert in high-sensitivity analytical technologies focused on immunoassays for biotechnological applications, Cygnus’ products and services have been used by nearly all major biopharmaceutical companies for more than 25 years.

As the exclusive distributor of Cygnus in China, Beijing XMJ Technology Co., Ltd. has established long-term and stable cooperative relationships with numerous well-known domestic pharmaceutical companies and CRO/CMO enterprises. Over the years, XMJ’s products and services have helped many enterprises accelerate the R&D phase, improve drug quality, purity and safety, optimize R&D processes more efficiently, shorten time-to-market for products, and reduce QC costs. If you are interested in the above-mentioned products, please feel free to call XMJ’s customer service hotline at 400-050-4006 or visit the website www.gq44.cn to learn more information.


.png) 京公網(wǎng)安備 11010802028692號(hào)
京公網(wǎng)安備 11010802028692號(hào)