BOUTIQUEResouces
Protein-based drugs, such as monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), are advancing at a rocket-like pace due to their excellent therapeutic efficacy and targeted action. While these drugs bring health benefits to patients, they also impose greater pressure on pharmaceutical R&D personnel. Owing to their instability under various environmental exposure conditions, pharmaceutical scientists are required to conduct continuous monitoring of drug quality to meet the needs of production and clinical applications. During the preparation, production, transportation, and storage processes, proteins may be subjected to multiple stresses such as freeze-thaw cycles, high temperatures, and agitation—raising concerns about the increased formation of protein aggregates. Protein aggregation affects drug safety; in severe cases, it may even increase the immunogenicity risk to patients. Fortunately, size exclusion chromatography (SEC) has become the "gold standard" as an effective detection method for monitoring protein aggregates in protein-based drugs. We used the ZF-LC-0001 size exclusion chromatography-high performance liquid chromatography (SEC-HPLC) universal mobile phase to analyze monoclonal antibodies and antibody-drug conjugates, and achieved highly satisfactory results.
Currently, SEC-HPLC methods have been standardized. Researchers can select standard commercial chromatographic columns based on protein molecular weight for quantitative analysis, but the choice of mobile phase usually relies on experience. These mobile phases are generally salt-containing aqueous solutions, which are adjusted to a specific pH value before analysis. However, salt-containing mobile phases are prone to bacterial growth during SEC-HPLC analysis. Although adding 0.5-1 M sodium chloride to the mobile phase can exert a certain bacteriostatic effect, the effect is limited and may pose the risk of salting-out and instrument clogging. Some researchers also choose to add a small amount of sodium azide to the mobile phase to inhibit bacterial growth, but with the gradual tightening of chemical regulatory requirements, this method has become unfeasible.
In the R&D stage of protein drugs—such as the pre-formulation development stage—it is often necessary to conduct multi-formulation screening designs and comprehensive stress tests. These stress tests include high temperature, freeze-thaw cycles, light exposure, and agitation. The comprehensive design of the experiments leads to an increase in the number of samples to be analyzed; for example, a single head-to-head SEC-HPLC analysis may involve more than 200 samples (with N=3), resulting in a liquid chromatography analysis time of over 4 days. Typically, the analysis of a single sequence cannot be interrupted. If an unexpected interruption occurs, new calibration and system equilibration are required, and the data obtained before the interruption can only be used after quality assurance evaluation. To address this issue, ZF Biology has newly launched an SEC-HPLC universal mobile phase (Catalog No.: ZF-LC-0001), which supports continuous sample analysis and does not affect the analysis results within 10 days under normal conditions.
1. Comparison of Bacteriostatic Efficacy of SEC Mobile Phases
The mobile phase without Zhoufan Biology's proprietary formula used in this experiment was prepared in accordance with conventional formulations. The control group mobile phase and ZF-LC-0001 mobile phase (1×) were respectively filled into clean and transparent 1L mobile phase bottles. Photos were taken for sample retention on days 0, 1, 2, 5, and 10, and the recorded images are shown in Figure 1. Compared with the control group mobile phase, the ZF-LC-0001 mobile phase exhibited excellent bacteriostatic efficacy. The results showed that visible foreign substances appeared in the control group mobile phase on the 1st day, and the phenomenon worsened on the 2nd day. The samples taken on the 5th day and the 10th day showed similar states when photographed. In contrast, the ZF-LC-0001 mobile phase remained clear and transparent until the 10th day. The mobile phases retained during this sampling period were used for the subsequent experimental analysis.

2. Intraday and Interday Precision Analysis
The mobile phase ZF-LC-0001, which had been stored in a regular analytical environment for 10 days, was used to perform intraday and interday precision analysis on the sample of recombinant anti-human ER2 antibody factor monoclonal antibody (see Figure 2). The data demonstrated that the mobile phase still maintained excellent intraday and interday precision after 10 days of storage: [Retention time (Time) and total peak area (mAU) are shown in Figure A and Figure C respectively], which were used to distinguish the intraday precision between different sequences. The latter 3 samples were injected in one run on the 2nd day, and the interday precision was compared with the samples injected in the second run on the 1st day (Retention time and total peak area are shown in Figure B and Figure D respectively).
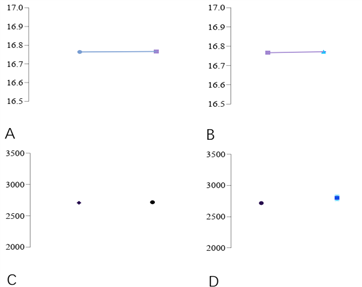
Figure 2. Intraday and Interday Precision of Zhoufan Biology's ZF-LC-0001 Mobile Phase
3. High Data Accuracy
In the process of analyzing 8 injection volume gradients of the recombinant anti-human ER2 antibody factor monoclonal antibody using ZF-LC-0001, the correlation coefficient R2 reached 0.9999. The data indicates that within the maximum analytical limit of the instrument, changes in the injection volume gradient have little impact on the accuracy of the data.
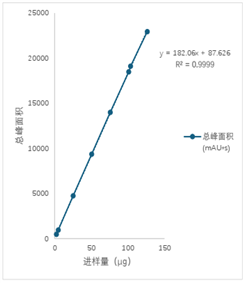
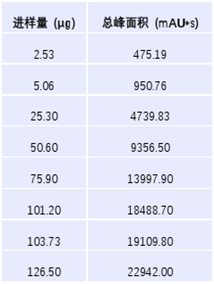
Figure 3. Linearity, Injection Volume and Total Peak Area Data of Zhoufan Biology's ZF-LC-0001 Mobile Phase
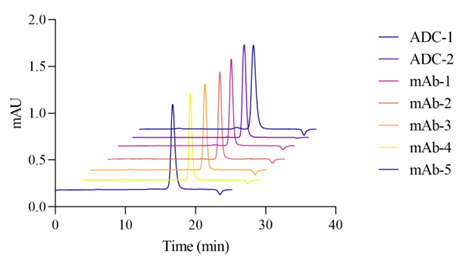
Size exclusion chromatography (SEC) analysis was performed on 5 monoclonal antibodies (mAbs) and 2 antibody-drug conjugates (ADCs) using ZF-LC-0001. All 7 protein drugs were diluted to 1 mg/mL with pure water and injected uniformly. The results, as shown in Figure 4, indicate that the ZF-LC-0001 mobile phase can achieve universal analytical effects both in common monoclonal antibody drugs and antibody-drug conjugates with strong hydrophobicity. Moreover, for low-concentration injections, protein aggregates and monomers still exhibit excellent resolution.
4. Broad Sample Compatibility
5. Improved Analytical Chromatograms
In addition, compared with conventional mobile phases, the use of ZF-LC-0001 yields more optimal peak shapes (as shown in Figure 5). The peaks exhibit narrower widths and reduced tailing—this effectively eliminates the need to add organic solvents to the mobile phase for tailing reduction, thereby further avoiding the risk of salting-out in the solution (which clogs the analytical system) and unexpected termination of the analysis sequence.
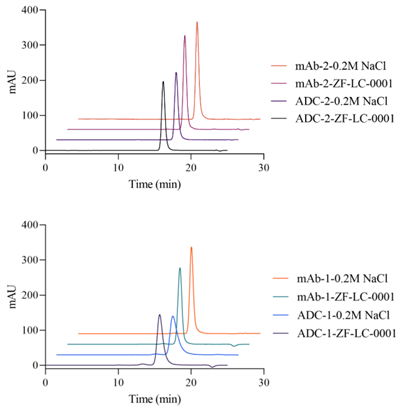
Figure 5. Comparison of SEC Chromatograms Between ZF-LC-0001 and Conventional High-Salt Mobile Phases (Samples: 2 Types of mAbs and 2 Types of ADCs)
Summary
In summary, the newly launched SEC-HPLC universal mobile phase (Catalog No.: ZF-LC-0001) from Zhoufan Biology offers the following prominent advantages:
· Superior antibacterial performance: Effectively protects valuable instruments and high-cost consumables from damage during the analysis process;
· Excellent stability: Maintains satisfactory intraday and interday stability as well as favorable linearity data even after being placed under analytical conditions for 10 days;
· Broad sample compatibility: Suitable for the analysis of various protein-based drugs, including monoclonal antibodies (mAbs), antibody-drug conjugates (ADCs), recombinant proteins, and peptides (data for this part is not shown);
· Improved chromatogram peak shape: Optimizes peak tailing issues;
· User-friendly: Non-toxic to the human body and environmentally friendly.
Product Ordering Information:
Item Number
Product Name
Specification
ZF-LC-0001
SEC Universal Mobile Phase Kit(10×)
1000 ml
Trial Application:
Applications for the SEC Universal Mobile Phase Trial Kit (100ml) are now open! If you are interested in the aforementioned product, please follow the "Ximeijie WeChat Official Account", send the keyword "SEC", and fill out the questionnaire to receive a free trial opportunity. Limited quantity, available on a first-come, first-served basis!

Hangzhou ZF Biotechnology Co., Ltd.
Founded in 2018, Hangzhou ZF Biotechnology Co., Ltd. boasts a highly efficient R&D team and is a company focusing on the field of biotechnology. The company has rich experience and technical advantages in areas such as protein drug process development and optimization, and molecular diagnostics. Our products include commercial capillary gel electrophoresis (CGE) assay kits, high-performance liquid chromatography (HPLC) ion exchange assay kits, cell culture reagents, molecular diagnostic reagents, etc. These products are widely used in fields like life science research and drug development. We have established close cooperative relationships with many well-known research institutions and enterprises to jointly promote the development and application of biotechnology.

Beijing XMJ Technology Co., Ltd.
As the general agent of ZF Biology, Beijing XMJ Technology Co., Ltd. has always adhered to a professional and rigorous attitude to provide customers with high-quality products and services. If you are interested in the aforementioned products, you are welcome to send email to info@xmjsci.com or visit the website www.gq44.cn to learn more about the product information.
Reference:
Liu JZ, Li L, Fang WJ*. A novel size exclusion chromatography method for the analysis of monoclonal antibodies and antibody-drug conjugates by using sodium iodide in the mobile phase. Pharm Res, 2024, in press.


 京公網(wǎng)安備 11010802028692號
京公網(wǎng)安備 11010802028692號