BOUTIQUEResouces
Since Mirus launched RevIT? AAV Enhancer in late July 2023, the integration of RevIT? AAV Enhancer has enabled a 1.2- to 4.9-fold increase in genome titers of AAV vectors across different serotypes. It has also improved vector infectivity to a certain extent, making it possible to further reduce upstream costs in AAV production and lowering the cost per dose of gene therapies. Owing to its ease of flexible integration into existing AAV manufacturing workflows—being compatible not only with Mirus’ TransIT-VirusGEN® GMP transfection reagent but also with other transfection reagents (e.g., PEI)—RevIT? AAV Enhancer has gained widespread recognition and acclaim from AAV clients both domestically and internationally. Additionally, the GMP-grade reagent has been launched as scheduled for large-scale GMP-compliant AAV production.
The GMP-grade RevIT? AAV Enhancer was released on September 30, 2024. Manufactured in a facility certified under the ISO 13485 system, it complies with ISO 20399 and USP <1043> standards. Since its launch on July 28, 2023, RevIT? AAV Enhancer has been proven to increase viral titers by at least 2- to 4-fold across different AAV serotypes, cell culture media, and transfection platforms. While maintaining high-quality titers, it allows for reduced plasmid DNA usage, thereby significantly cutting down AAV vector production costs.
Product Details
· Product Name: RevIT? GMP AAV Enhancer
· Launch Date: September 30, 2024
· Product Catalog No.: MIR 8200-GMP
· Product Specification: 200mL (Liquid)
For more data and detailed information about RevIT? AAV Enhancer, please refer to the WeChat article titled "New Product Launch | RevIT? AAV Enhancer: Another Powerful Tool to Boost AAV Yield!"
Product Specifications for RevIT AAV Enhancer and TransIT-VirusGEN Transfection Reagent
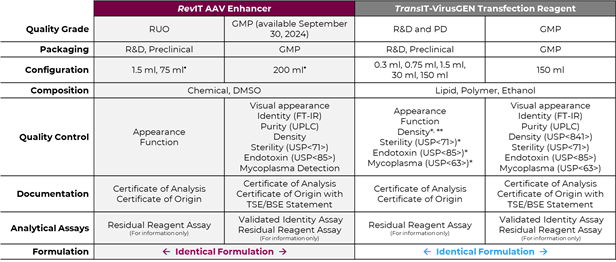
ü Tubes/Bottles Made of HDPE
ü Applicable Only to Reagents with Specifications of 30 mL and 150 mL
ü Applicable Only to Reagents with Specification of 150 mL
GMP Support Details
· Residual Reagent Assay: Residual Detection
· Regulatory Support Package: Regulatory Support Documents
· Product Safety Risk Assessment | Supplemental Information: Product Safety Risk Assessment | Supplementary Information
· Manufactured and Tested in Alignment with Mirus Standards
· ISO 13485 Quality Management (QM)
Mirus Bio GMP Quality Standards
ISO 13485 Validation System
The ancillary materials developed and supplied by Mirus Bio can be used in Research Use Only (RUO) and GMP-compliant manufacturing processes for cell and gene therapies.
Mirus Bio’s Quality Management System (QMS) is ISO 13485-certified, enabling technology transfer and distribution of GMP-grade ancillary materials.
Mirus Bio develops and implements a customer-centric Quality Management System (QMS). As a trustworthy partner, it upholds its commitment to meeting the needs of customers in the cell and gene therapy field.
The following guidance documents are the quality standard documents referenced by Mirus Bio in establishing its Quality Management System:
· ISO 13485:2016, Medical devices – Quality management systems – Requirements for regulatory purposes
· ISO 20399:2022, as ancillary materials present during the production of cellular therapeutic products
USP<1043>, with respect to Tier 2 ancillary materials for cell, gene and tissue-engineered product

More Customer Case Sharing
Latest Online Webinar: Pairing a Novel Enhancer with Transfection Reagents to Enable High-Titer rAAV Production
Speaker Information:
· Florian Dziopa – Director of Upstream Process at MeiraGTx. Over the past six years at MeiraGTx, he has been dedicated to optimizing AAV production platforms based on transient transfection processes using suspension HEK293 cells.
· René Gantier – Senior Director of Bioprocess Applications & R&D at Repligen. He has over 20 years of experience in product development and process purification.
· Becky Reese – R&D Specialist at Mirus Bio
Webinar Access Online Viewing (Link)
· Learn how to optimize and enhance viral vector manufacturing processes;
· Understand how VirusGEN® transfection reagents drive comprehensive yield improvements across workflows involving different transfection protocols and AAV serotypes;
· Explore strategies to reduce the cost per dose of cell and gene therapies from the perspective of manufacturing process optimization;
· Simplify the entire process from technical evaluation to commercial manufacturing.
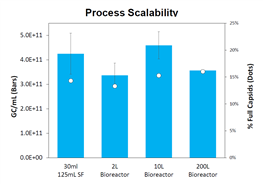
Poster Data: Optimizing Key Parameters for AAV5 Clinical Manufacturing: Enhancing Process Productivity and Drug Substance Quality (Link)

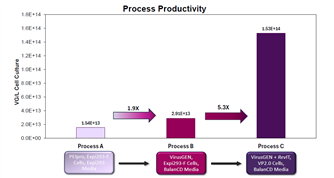
In this poster, Bridgebio shared data on viral titer and infectivity (left panel) for the AAV5 serotype during scale-up from shake flask to 200L (125mL shake flask → 2L → 10L → 200L), using TransIT-VirusGEN® transfection reagent and RevIT? AAV Enhancer. Compared with the previous process (Process A: PEIPro transfection + Expi293 cells/media), the processes using TransIT-VirusGEN® transfection reagent as a replacement (Process B: TransIT-VirusGEN® transfection reagent + Expi293 cells + BalanCD medium; Process C: TransIT-VirusGEN® transfection reagent + VP2.0 cells + BalanCD medium) achieved higher viral titers (right panel) and lower residual host DNA and plasmid DNA.
Relevant Product Information
|
Product Name |
Specification |
Item Number |
|
RevIT? AAV Enhancer |
1.5 ml |
MIR 8000 |
|
10 x 1.5 ml |
MIR 8006 |
|
|
75 ml |
MIR 8080 |
|
|
NEW! RevIT? GMP AAV Enhancer |
200 ml |
MIR 8200-GMP |
|
VirusGEN® AAV Transfection Reagent及RevIT? AAV Enhancer |
For 1L of Culture |
MIR 8007 |
|
For 10L of Culture |
MIR 8008 |
|
|
TransIT-VirusGEN®Transfection Reagent |
0.3 ml |
MIR 6703 |
|
0.75 ml |
MIR 6704 |
|
|
1.5 ml |
MIR 6700 |
|
|
5 x 1.5 ml |
MIR 6705 |
|
|
10 x 1.5 ml |
MIR 6706 |
|
|
30 ml |
MIR 6720 |
|
|
TransIT-VirusGEN® GMP grade Transfection Reagent |
150 ml |
MIR 6845-GMP |
|
VirusGEN® AAV Transfection (Kit) |
1 kit for 1 L of cell culture |
MIR 6750 |
|
VirusGEN® GMP grade AAV Transfection (Kit) |
1 Kit - for 50 L of cell culture |
MIR 6815-GMP |
|
VirusGEN® LV Transfection (Kit) |
1 kit for 1 L of cell culture |
MIR 6760 |
|
VirusGEN® GMP grade LV Transfection (Kit) |
1 Kit - for 50 L of cell culture |
MIR 6825-GMP |
New Product Express
The TransIT®-AAViator Transfection System is developed based on Mirus Bio's patented and mature polymer and lipid technology platform. Compared with transfection reagents based solely on polymers (PEI), it can further increase the yield of AAV vectors and allows a 50% reduction in plasmid DNA usage. The TransIT-AAViator System consists of the new TransIT-AAViator Transfection Reagent and RevIT? AAV Enhancer. When used in combination, they significantly optimize upstream transfection—a key step in AAV manufacturing—maximizing AAV titers. Stay tuned for more details.
Founded in 1995, Mirus has adhered to providing optimized methods and efficient tools for gene delivery. With over 25 years of dedicated efforts in the field of transfection, it has achieved multiple technological breakthroughs and has become a global leader and trusted brand in the field of nucleic acid delivery. Mirus' team of scientists continues to push the boundaries of transfection technology, launching VirusGEN® transfection reagents and RevIT? enhancers, which further increase AAV/LV yields, help promote cost reduction and efficiency improvement of biotherapeutic drugs, and empower customers in the CGT field.

Beijing XMJ Technology Co., Ltd. is the exclusive distributor of Mirus in China. Adhering to a professional and rigorous attitude, it provides customers with high-quality products and services. Mirus' best-selling transfection products are always available in stock. If you are interested in the aforementioned products, please feel free to send email to info@xmjsci.com or visit the website www.gq44.cn for more product information.



 京公網(wǎng)安備 11010802028692號
京公網(wǎng)安備 11010802028692號