BOUTIQUEResouces
Cygnus Protein A Mix-N-Go? Series ELISA Kits
Cygnus Protein A Mix-N-Go? Series ELISA Kits are specifically designed for detecting residues of most natural and recombinant Protein A. They adopt a revolutionary one-step sample processing procedure. Compared with traditional Protein A ELISA methods, the Mix-N-Go? Series ELISA Kits simplify experimental procedures, shorten assay time, and simultaneously improve method reliability.
Advantages of Protein A Mix-N-Go? Kits:
· No boiling or centrifugation steps required — reducing the use and calibration of equipment.
· Robust method — ensuring irreversible dissociation of Protein A from IgG.
· Rapid access to test results — assay time is 2 hours shorter than that of other kits.
· Higher precision — fewer steps lead to less error.
· All reagents required for detection and pre-prepared standards are provided in the kit.
Comparison of Operational Procedures: Cygnus Protein A Mix-N-Go? vs. Protein A Detection Kits of Other Brands
|
Feature |
Cygnus Protein A Mix-N-Go? ELISA Kit |
Brand A |
Brand B |
|
Sample Handling |
|
|
|
|
Protein A-IgG irreversible dissociation? |
Yes |
No |
No |
|
Boiling required? |
No |
Yes |
Yes |
|
Centrifugation required? |
No |
Yes |
Yes |
|
Allowable IgG concentration in sample |
≤20 mg/mL |
1-15 mg/mL |
Must dilute to 50 μg/mL |
|
Total sample processing time |
10 min |
45 min |
54 min |
|
Step |
Cygnus Protein A Mix-N-Go? ELISA Kit |
Brand A |
Brand B |
|
Reagent Preparation |
All reagents are ready-to-use |
Require preparation to working concentration |
Require preparation to working concentration |
|
Reagent Prep Time |
None |
~10 min (diluting standards) |
~10 min (diluting standards) |
|
Reaction Method |
One-step reaction |
Multi-step reaction |
Multi-step reaction |
|
Wash Steps |
1 |
4 |
3 |
|
Total Hands-on Time |
1h 45min |
2h 5min |
3h 54min |
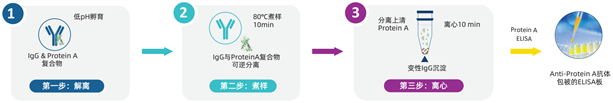
One-Step Protein A Mix-N-Go? Sample Processing Procedure

Three-Step Routine Protein A Sample Processing Procedure
Guide for Selecting Protein A Mix-N-Go? ELISA Kits
Cygnus Protein A Mix-N-Go ELISA Kits have been widely used in batch release testing for numerous commercial therapeutic monoclonal antibodies. They have also undergone specific testing and validation for compatibility with most commercial Protein A chromatography resins. To target Protein A ligands from different brand sources, Cygnus has designed and developed a variety of detection kits; the recommended item numbers are shown in the table below.
|
Protein A Resin |
Resin Manufacture |
Recommended Cygnus Protein A
Mix-N-Go? Kit Item No. |
Correction Factor* |
|
Amsphere? A3 |
JSR Life Sciences® |
F740 |
Specialized Kit |
|
Amsphere? A+ |
JSR Life Sciences® |
F1075 New Launched |
Specialized Kit |
|
Eshmuno® A |
MilliporeSigma®/
MerckMillipore? |
F610 |
1 |
|
J.T.Baker® BAKERBOND®
PROchievA? Protein A |
Avantor® |
F965 |
Specialized Kit |
|
KanCapA? 3G Protein A |
Kaneka® |
F950 |
Specialized Kit |
|
MabSelect? |
Cytiva® |
F600 |
1 |
|
MabSelect? SuRe,
MabSelect? SuRe XL |
Cytiva® |
F610 |
1 |
|
MabSelect? PrismA? |
Cytiva® |
F610 |
1.6 |
|
Praesto? Jetted A50 |
Purolite® |
F610 |
1.3 |
|
TOYOPEARL® SuperA (R50) |
Tosoh Bioscience® |
F910 |
Specialized Kit |
*The Correction Factor accounts for calibration discrepancies caused by differences in the molecular weights of Protein A ligands. It must be applied to the Protein A concentration values calculated from the F610 standard curve to determine the residual Protein A concentration corresponding to the relevant resin.
Protein A Mix-N-Go? ELISA Kit Ordering Information
|
Product Name |
Item Number |
|
Protein A Mix-N-Go? ELISA Kit |
F600 |
|
Protein A Mix-N-Go? ELISA Kit for UnNatural Protein A Constructs |
F610 |
|
Protein A Mix-N-Go? ELISA for Amsphere? A3 Ligand |
F740 |
|
Protein A Mix-N-Go? ELISA for Amsphere? A+ Ligand New Launched |
F1075 |
|
Tosoh R50, R40 and R28 Protein A Mix-N-Go? ELISA |
F910 |
|
KANEKA KanCapA? 3G Protein A Mix-N-Go? ELISA |
F950 |
|
J.T.Baker® BAKERBOND® PROchievA? Protein A Mix-N-Go? ELISA |
F965 |

Cygnus Technologies, LLC. provides products and analytical methods for the biotechnology and biopharmaceutical industries, aiming to accelerate the R&D phase and enhance product quality. The bioprocess residual detection kits developed and manufactured by Cygnus are used to detect specific impurities from over 50 different expression systems. As an expert in high-sensitivity analytical technologies focused on immunoassays for biotechnological applications, Cygnus’ products and services have been utilized by nearly all major biopharmaceutical companies for more than 25 years.

As the exclusive distributor of Cygnus in China, Beijing XMJ Technology Co., Ltd. has established long-term and stable cooperative relationships with numerous well-known domestic pharmaceutical enterprises and CRO/CMO companies. Over the years, XMJ's products and services have helped many enterprises accelerate the R&D phase, improve drug quality, purity and safety, optimize R&D processes more efficiently, shorten time-to-market for products, and reduce QC costs. If you are interested in the aforementioned products, please feel free to send email to info@xmjsci.com or visit the website www.gq44.cn for more information.


.png) 京公網(wǎng)安備 11010802028692號
京公網(wǎng)安備 11010802028692號