BOUTIQUEResouces
As a safety test item for biopharmaceuticals, endotoxin is a mandatory test item required by regulations. All pharmaceutical manufacturing processes impose strict controls on endotoxins (≤ 5 EU/kg body weight), making endotoxin removal a crucial step in biopharmaceutical process control. The current challenges in endotoxin removal include high time consumption; meanwhile, the removal process may also lead to product loss and introduction of toxic substances. Therefore, endotoxin removal has long been a 困擾 (plague) for many enterprises, especially during the large-scale production stage using Escherichia coli (E. coli).
The main methods for endotoxin removal include ultracentrifugation, ion exchange chromatography, and affinity chromatography. In large-scale production processes, the latter two methods are primarily adopted for endotoxin removal. Ion exchange chromatography works by binding negatively charged endotoxins; however, it exhibits less-than-ideal performance in removing endotoxins from plasmid DNA or acidic proteins. For this reason, the industry currently prefers affinity chromatography for endotoxin removal. Affinity chromatography offers several advantages: it can specifically bind endotoxins, results in minimal final product loss (compared to ion exchange chromatography), has a wide range of applications (including plasmid DNA and proteins), and avoids the introduction of toxic substances.
This article will recommend Etoxiclear? chromatography medium from Astrea Bioseparations (UK). It is a high-performance, non-ion-exchange, synthetic affinity chromatography adsorbent that enables cost-effective and efficient endotoxin removal. Supporting GMP-compliant production, Etoxiclear? is available in two product forms—gel media and single-use pre-packed columns—to meet diverse process requirements.
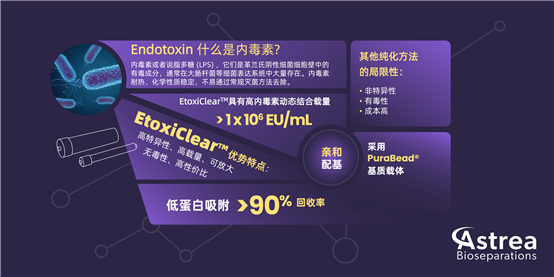
What is EtoxiClear? Affinity Chromatography?
Affinity chromatography is a specialized protein purification method that achieves separation by leveraging the specific interactions between biomolecules. EtoxiClear? Affinity Chromatography exhibits high selectivity and high resolution for endotoxins, enabling more effective endotoxin removal. The advantages of affinity chromatography lie in its high specificity and high purification efficiency, making it suitable for the production of biopharmaceuticals that demand extremely high purity. While ion exchange chromatography can control endotoxin levels to a certain extent, individual projects may still face the risk of endotoxin exceeding the standard. EtoxiClear? is an exceptionally unique endotoxin removal product on the market. Compared with ion exchange chromatography methods, the advantages of EtoxiClear? include:
· High Specificity: Specifically designed for endotoxin removal, enabling targeted elimination of endotoxins.
· High Loading Capacity: Boasts a high dynamic binding capacity for endotoxins (> 1,000,000 EU/ml).
· Cost-Effective and Scalable: Offers economic efficiency and supports process scale-up.
· Broad pH Compatibility Range: Functions stably within the pH range of 4.0 - 8.0.
· Compliant with GMP Material Requirements: Comes with comprehensive regulatory registration documentation.
· Low Protein Binding Rate: Typically achieves a target protein recovery rate of > 90%.
· Highly Stable, Synthetic, and Non-Toxic Affinity Ligand: Features a man-made ligand that is stable, non-toxic, and reliable.
· Two Product Forms (Pre-Packed Columns and Resins): Available in both pre-packed columns and resin formats to meet diverse application needs.
· Strong Alkali Resistance of Resins: Withstands cleaning-in-place (CIP) using 0.5M - 1M NaOH.
Performance Data of EtoxiClear? Endotoxin Affinity Resins
Scalability
EtoxiClear? has been proven effective in removing endotoxins from purified immunoglobulin solutions at both laboratory scale (5mL column) and industrial scale (785mL column).
|
Column Volume |
Endotoxin Starting Concentration (EU/mL) |
Loading Volume (mL) |
Total Endotoxin Load (EU) |
Endotoxin Concentration After Purification (EU/mg protein) |
|
5ml |
472 |
10 |
4,720 |
0.18 |
|
1600ml |
472 |
10 |
755,200 |
0.08 |
Sample Loading: IgG Protein Solution with Similar Initial Endotoxin Concentration (~5 mg/mL)
High Processing Capacity
EtoxiClear? is available in two product forms—resins and pre-packed columns—to meet diverse experimental requirements. The table below can be used to estimate the column size required for processing samples of different scales.
|
EtoxiClear? Purification Column |
Flow Rate (L/hr) |
||
|
Column Volume |
Diameter (cm) |
Bed Height (cm) |
|
|
785ml |
7 |
10 |
4.6 |
|
1.5L |
10 |
10 |
9.4 |
|
3.14L |
20 |
10 |
37.7 |
Specification: EvolveD? Column, 5-Minute Retention Time
Endotoxin Removal Data for Commonly Used Buffers
EtoxiClear? can be used for endotoxin removal in various buffers. The table below shows the endotoxin removal efficiency across different buffers commonly used in cell culture applications. Even with a relatively low initial endotoxin concentration (~20 EU/mL), a high endotoxin removal rate can still be achieved.
|
Buffer |
Initial Endotoxin Concentration (EU/mL) |
Loading Volume (mL) |
Total Endotoxin Load (EU) |
Endotoxin Removal (EU/mL) |
|
PBS |
23 |
300 |
6900 |
< 0.05* |
|
Earles (EBSS) |
16 |
400 |
6400 |
< 0.05* |
|
Hanks (HBSS) |
23 |
425 |
9775 |
≤ 0.05* |
Equal to or below the detection limit
High Endotoxin Clearance Rate
EtoxiClear? exhibits a high endotoxin clearance rate and enables excellent protein recovery from a variety of protein samples, such as the human serum albumin (HSA) and immunoglobulin (IgG) solutions (~9 mg/mL each) as shown in the table below.
|
Sample |
Endotoxin Concentration (EU/mg protein) |
Protein Recovery (%) |
|
HSA Load |
23.6 |
- |
|
HSA Flowthrough |
0.06 |
100 |
Endotoxin Clearance Rate of HSA Sample: 99.7%
|
Sample |
Endotoxin Concentration (EU/mg protein) |
Protein Recovery (%) |
|
IgG Load |
8.3 |
- |
|
IgG Flowthrough |
0.09 |
100 |
Endotoxin Clearance Rate of IgG Sample: 98.9%
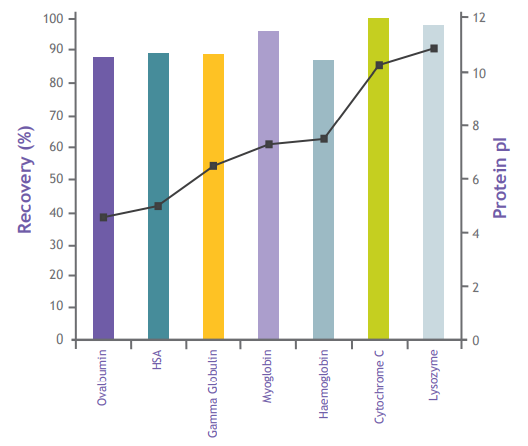
Low Protein Binding Rate
Unlike ion exchange adsorbents, EtoxiClear? exhibits extremely low protein binding properties. It is not limited by the isoelectric point range of proteins and enables high protein recovery (typically over 90%), as shown below:
EtoxiClear? Technical Specifications
|
Property |
Specification / Description |
|
Ligand: |
Synthetic chemical ligand (Patented) |
|
Average Particle Size: |
100±10 μm |
|
Matrix: |
6% cross-linked near-monodisperse agarose (PuraBead® 6XL) |
|
Dynamic Endotoxin Binding Capacity: |
>1,000,000 EU/mL |
|
Maximum Operating Flow Rate: |
Up to 400 cm/h (EtoxiClear 5 mL EvolveR Column) |
|
Recommended Operating Flow Rate: |
Up to 200 cm/h (Column size dependent) |
|
Working pH: |
pH 4.0 ~ pH 8.0 |
|
Chemical Stability: |
Stable in all common buffers and solutions |
|
Cleaning: |
0.5M ~ 1.0M NaOH |
|
Storage Conditions: |
2~30°C, 20% ethanol / 80% 0.1M NaCl (v/v) |
Product Ordering Information
|
Item Number |
Product Name |
Specification |
|
3250-00010 |
EtoxiClear (10 mL) |
10mL |
|
3250-00025 |
EtoxiClear (25 mL) |
25mL |
|
3250-00100 |
EtoxiClear (100 mL) |
100mL |
|
3250-00500 |
EtoxiClear (500 mL) |
500mL |
|
3250-01000 |
EtoxiClear (1 L) |
1L |
|
6646 |
EtoxiClear? 4 x 1 mL |
4x1mL |
|
6647 |
EtoxiClear? 4 x 5 mL |
4x5mL |
|
6746 |
EtoxiClear® Column, 1 x 1 mL |
1 x 1 mL |
|
6747 |
EtoxiClear® Column, 1 x 5 mL |
1 x 5 mL |
|
4251-00005 |
EtoxiClear 5 mL Column |
5mL |
|
4251-00050 |
EtoxiClear 50 mL Column |
50mL |
|
AS025100-PC3250-G |
EtoxiClear 50 mL EvolveS |
50mL |
|
4520-PC3250 |
EtoxiClear?, 200 μL |
200μL |

Astrea Bioseparations Incorporated as a spin-off from the University of Cambridge in 1987, Astrea Bioseparations boasts over 30 years of experience in the research, development and manufacturing of chromatographic media. It stands as a world-class supplier of chromatographic media products and services. To date, more than 21 manufacturing processes utilizing Astrea's products have obtained approvals from the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
With 3 R&D and manufacturing facilities worldwide, Astrea Bioseparations focuses on providing industry-leading chromatographic media and technical services for the biopharmaceutical (large molecule) and Cell & Gene Therapy (CGT) sectors. The company's innovative nanofiber-based chromatographic technology addresses the low capacity and time-consuming drawbacks of traditional bioseparation tools, enabling faster, more environmentally friendly, and cost-effective purification processes. This fully aligns with the evolving demands of today's biotherapeutic innovation.

XMJ is the authorized distributor of Astrea Bioseparations in China, providing customers with comprehensive technical support and after-sales service. For more information, please feel free to send email to info@xmjsci.com or visit the official website at www.gq44.cn at any time.


.png) 京公網(wǎng)安備 11010802028692號
京公網(wǎng)安備 11010802028692號