BOUTIQUEResouces
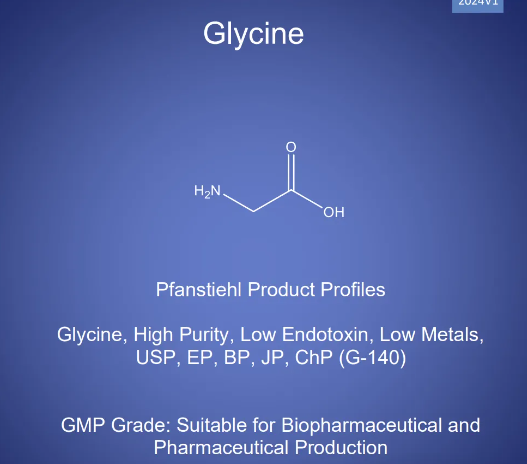
Glycine is the simplest amino acid in terms of structure, with its systematic name being aminoacetic acid and molecular formula C?H?NO?. At room temperature, it appears as white crystals or crystalline powder with a sweet taste. It is highly soluble in water and also dissolves in organic solvents such as ethanol and acetone, demonstrating excellent hydrophilicity. The glycine molecule contains both an acidic carboxyl group (-COOH) and a basic amino group (-NH?), enabling it to react with both acids and bases. It exhibits diverse ionization states under different pH environments and possesses outstanding buffering capacity.
Owing to these characteristics, glycine is widely used as a pharmaceutical excipient and offers numerous advantages, with the following applications:
· Buffering agent: The zwitterionic nature of glycine molecules allows them to stabilize the pH of solutions under different pH conditions, maintaining a stable formulation environment and ensuring that the active pharmaceutical ingredients are not affected by changes in acidity or alkalinity. This is crucial for pharmaceutical formulations sensitive to pH, such as biological products and enzymatic drugs.
· Solubilizing agent: With good hydrophilicity, glycine can interact with poorly soluble drug molecules, reducing the aggregation between these molecules. This enables the drugs to disperse in molecular or ionic form, increasing their solubility in solvents and facilitating the preparation of solutions from poorly soluble drugs.
· Stabilizing agent: In protein and peptide drug formulations, glycine can preferentially bind to surrounding water molecules, reducing interference with the hydration layer of drug molecules. It prevents drugs from inactivating due to aggregation or denaturation, effectively maintaining the stability of such biological macromolecular drugs.
However, glycine also has limitations when used as a pharmaceutical excipient. For instance, when applied in lyophilized formulations, it tends to exhibit abnormal crystal morphology; when used in combination with other excipients, it can alter the collapse temperature of the entire lyophilization system. Additionally, improper use of glycine for regulating the osmotic pressure of injectables may easily lead to excessively high osmotic pressure. Notably, glycine, as a pharmaceutical excipient, has extremely high purity requirements. Even trace impurities in glycine raw materials can trigger immune responses or toxic side effects in the human body after entry. Purifying glycine to meet injectable-grade standards is technically challenging and costly, which to a certain extent restricts its large-scale application in pharmaceutical formulations.
Adhering to its consistent philosophy of high quality, Pfanstiehl has grandly launched the injectable-grade Glycine (G-140) to meet the global biopharmaceutical industry's demand for high-quality glycine. This product features ultra-high purity, ultra-low endotoxins, and ultra-low metal residues. It undergoes individual testing for 29 types of metal ions, and additional quality indicators have been added for testing, including DNA, RNA, DNase, RNase, lipase, and nitrite. It complies with the regulations of multiple national pharmacopoeias (USP, EP, BP, JPE, ChP) and can be used in the production of biotherapeutic drugs and vaccines.
Product Information
· Product Name: Glycine, High Purity, Low Endotoxin, Low Metals, USP, EP, BP, JPE, ChP (G-140)
· Molecular Formula: C?H?NO?
· Molecular Weight: 75.07 g/mol
· CAS Number: 56-40-6
· Product Item Number: G-140
Product Features
· Ultra-high purity, ultra-low endotoxins, ultra-low microbial content, and ultra-low residual metal impurities
· Additional quality indicator tests: DNA, RNA, DNase, RNase, lipase, and nitrite
· Compliant with multiple national pharmacopoeias: USP, EP, BP, JPE, ChP
· Compliant with ICH Q3D guidelines
· Compliant with GMP standards
· In the process of pharmaceutical excipient registration with US DMF and China CDE
Product Applications
· Buffering agent
· Stabilizing agent
· Solubilizing agent
If you are interested in Pfanstiehl's Injectable-Grade Glycine (G-140), please contact Beijing XMJ Technology Co., Ltd., the authorized distributor of Pfanstiehl in China, to obtain the following detailed product overview documents and test samples.
品目錄表Eng._2__01.png)


.png) 京公網(wǎng)安備 11010802028692號(hào)
京公網(wǎng)安備 11010802028692號(hào)