BOUTIQUEResouces
Warm Congratulations! The CDE Registration Number F20200000338 for Pfanstiehl’s Injectable-Grade Histidine (H-116) Has Been Activated!
Pfanstiehl is welcoming a spate of good news in the New Year. Following the recent activation of its injectable-grade arginine, the much-anticipated CDE registration number F20200000338 for injectable-grade Histidine (H-116) has finally been activated and upgraded to "Category A".

Since its launch in 2020, Pfanstiehl’s injectable-grade Histidine (H-116) has adhered to the brand’s consistent high-quality philosophy. It features ultra-high purity, ultra-low endotoxins, and ultra-low residual metals, with individual testing for 29 types of metal ions. Compliant with the requirements of multiple pharmacopoeias (USP, EP, JPE, ChP), it can be used in the production of biotherapeutic drugs and vaccines.
Pfanstiehl’s injectable-grade Histidine will undergo a comprehensive quality specification upgrade again in March 2025:
· The quality control (QC) specifications for 29 metal ions will be further narrowed down to ppb-level limits;
· In line with FDA and EMA guidelines on nitrites, QC testing for 10 types of nitrites will be newly added;
· Controls for various enzymes, including DNA, RNA, DNase, RNase, lipase, phosphatase, and protease, will be incorporated.
These upgrades will further reduce potential negative impacts—introduced by histidine—on protein stability and the degradation of other excipients (e.g., Tween).
Product Information
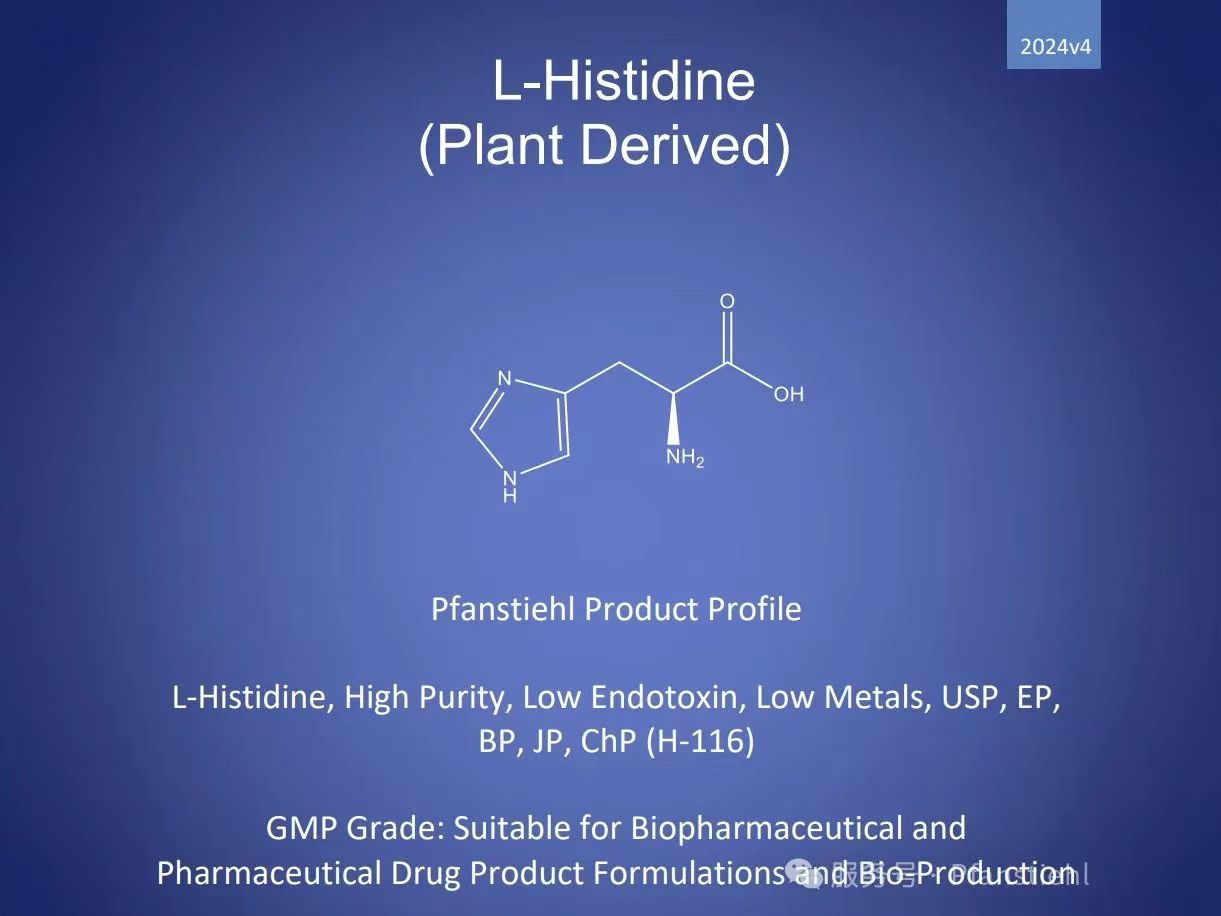
· Product Name: L-Histidine, High Purity, Low Endotoxin, Low Metals, USP, EP, BP, JP, ChP (H-116)
· Molecular Formula: C?H?N?O?
· Molecular Weight: 155.15 g/mol
· CAS Number: 71-00-1
· Product Code: H-116
Product Features
· Ultra-high purity, ultra-low endotoxins, ultra-low microbial content, and ultra-low residual metal impurities
· Newly added testing for DNA, RNA, DNase, RNase, lipase, and nitrites in quality specifications
· Compliant with multiple pharmacopoeias: USP, EP, BP, JPE, ChP
· Compliant with ICH Q3D guidelines
· Compliant with GMP standards
Product Application
· Buffer
If you are interested in Pfanstiehl’s Injectable-Grade L-Histidine (H-116), please contact Beijing XMJ Technology Co., Ltd.—the authorized distributor of Pfanstiehl in China—to obtain the detailed product overview documents listed below. You are also welcome to contact us to request test samples.
The product series of Pfanstiehl Inc. is as follows. You are welcome to contact Beijing XMJ Technology Co., Ltd. to obtain free samples in 100g packaging.
Warm Congratulations! The CDE Registration Number F20200000338 for Pfanstiehl’s Injectable-Grade Histidine (H-116) Has Been Activated!
Pfanstiehl is welcoming a spate of good news in the New Year. Following the recent activation of its injectable-grade arginine, the much-anticipated CDE registration number F20200000338 for injectable-grade Histidine (H-116) has finally been activated and upgraded to "Category A".
Since its launch in 2020, Pfanstiehl’s injectable-grade Histidine (H-116) has adhered to the brand’s consistent high-quality philosophy. It features ultra-high purity, ultra-low endotoxins, and ultra-low residual metals, with individual testing for 29 types of metal ions. Compliant with the requirements of multiple pharmacopoeias (USP, EP, JPE, ChP), it can be used in the production of biotherapeutic drugs and vaccines.
Pfanstiehl’s injectable-grade Histidine will undergo a comprehensive quality specification upgrade again in March 2025:
· The quality control (QC) specifications for 29 metal ions will be further narrowed down to ppb-level limits;
· In line with FDA and EMA guidelines on nitrites, QC testing for 10 types of nitrites will be newly added;
· Controls for various enzymes, including DNA, RNA, DNase, RNase, lipase, phosphatase, and protease, will be incorporated.
These upgrades will further reduce potential negative impacts—introduced by histidine—on protein stability and the degradation of other excipients (e.g., Tween).
Product Information
· Product Name: L-Histidine, High Purity, Low Endotoxin, Low Metals, USP, EP, BP, JP, ChP (H-116)
· Molecular Formula: C?H?N?O?
· Molecular Weight: 155.15 g/mol
· CAS Number: 71-00-1
· Product Code: H-116
Product Features
· Ultra-high purity, ultra-low endotoxins, ultra-low microbial content, and ultra-low residual metal impurities
· Newly added testing for DNA, RNA, DNase, RNase, lipase, and nitrites in quality specifications
· Compliant with multiple pharmacopoeias: USP, EP, BP, JPE, ChP
· Compliant with ICH Q3D guidelines
· Compliant with GMP standards
Product Application
· Buffer
If you are interested in Pfanstiehl’s Injectable-Grade L-Histidine (H-116), please contact Beijing XMJ Technology Co., Ltd.—the authorized distributor of Pfanstiehl in China—to obtain the detailed product overview documents listed below. You are also welcome to contact us to request test samples.
品目錄表Eng._1__01.png)

Beijing XMJ Technology Co., Ltd. is the authorized distributor of Pfanstiehl in China, providing customers with comprehensive technical support and after-sales services. For more information, please feel free to send email to info@xmjsci.com or visit the official website at www.gq44.cn.


.png) 京公網(wǎng)安備 11010802028692號
京公網(wǎng)安備 11010802028692號