BOUTIQUEResouces
The Definition of Blank Control and Its Application in ELISA Assays
A blank control refers to a type of negative control designed in experimental setups, aiming to monitor non-specific background signals and provide a reference for baseline absorbance values. In Enzyme-Linked Immunosorbent Assays (ELISA), the introduction of a blank control enables effective identification and subtraction of background noise from sample readings, thereby improving the accuracy of detection results. However, in multi-analyte ELISA assays, this standardization method may not be applicable due to their inherent characteristic of high background signals. This article will explore in detail why the use of blank control is generally not recommended in Cygnus' ELISA assays.
The Impact of Background Value Subtraction on Multi-Analyte ELISA Detection Results
In multi-analyte ELISA assays, such as Host Cell Protein (HCP) ELISA, the background signal is usually high due to the complexity of HCP antibodies. This causes the baseline absorbance values (OD values) of such assays to be generally higher than those of simple single-analyte assays. From a statistical perspective, subtracting the higher baseline OD values from sample readings may increase the degree of data variation, thereby affecting the accuracy and reliability of the results.
The following example illustrates the impact of background value subtraction on the coefficient of variation (CV). In Table 1, the average OD value of Standard A is 0.100, and that of Standard B is 0.150. When the CV is calculated by subtracting the OD value of the blank control from the original readings, the CV value increases significantly—resulting in the CV% of Standard B rising from 6.7% to 20%.
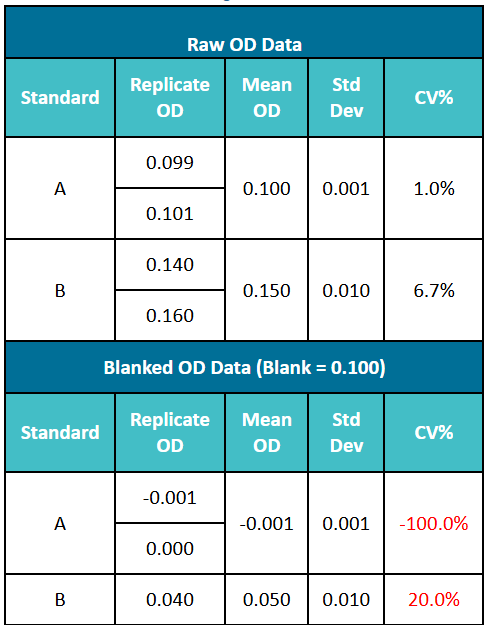
Table 1. The Impact of Background Value Subtraction on the Coefficient of Variation
The higher the detected background value, the greater its impact on the percentage of the coefficient of variation (CV%).
Table 2 shows the average coefficient of variation (CV%) of 8 different ELISA kits across 10 random datasets. The CV% calculated based on the original OD values ranges from 10% to 20%, while the CV% of the same data after blank control correction exceeds 20%. In all cases, the CV% after blank control correction is higher than that of the uncorrected data. In practical applications, the criteria for analytical data release usually require that the CV% of duplicate tests does not exceed 20%. The above difference may directly cause the analytical results to fail to meet the precision requirements. In the long run, the introduction of blank control will directly lead to the test results failing to meet the release requirements.
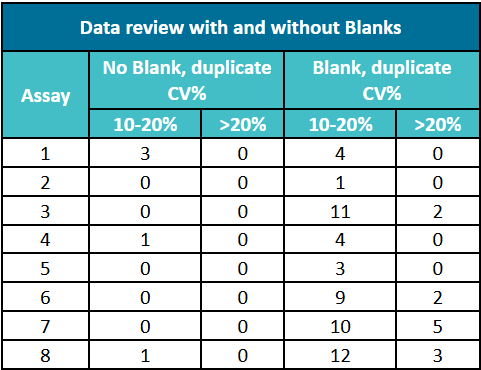
Table 2. Comparison of CV% Values Between Blank Control-Corrected Data and Uncorrected Data
From a statistical perspective, the higher the OD value of the blank control, the greater the likelihood of such issues occurring.
Due to the above reasons, Cygnus Technologies is committed to developing and providing ELISA kits that do not require background correction. Throughout the development, analysis, and testing processes of our marketed ELISA kits, the blank control correction step has never been incorporated into the batch release workflow. Instead, we use the 0 ng/mL standard to set the Y-axis intercept and determine the lower limit of the standard curve. Therefore, if you include the blank control correction step in your experimental workflow, the percentage of the coefficient of variation (CV) obtained from your detection is likely to be higher than the results recorded by the Cygnus Quality Control team. Cygnus provides a corresponding Qualification Summary (QS) document for each type of detection kit. If needed, please click here to fill in the information to request it, or contact XMJ, the general agent of Cygnus in China, directly. If you encounter any problems while using Cygnus HCP ELISA kits, our technical support team will be dedicated to providing you with solutions and suggestions.

Cygnus Technologies has specialized in providing products and analytical methods for the biotechnology and biopharmaceutical industries for over 25 years, with the aim of accelerating the R&D phase and enhancing product quality. Cygnus develops and manufactures bioprocess residual kits, which are used to detect specific impurities in more than 50 different expression systems. As an expert in high-sensitivity analytical technologies focused on immunoassays for biotechnological applications, Cygnus' products and services are utilized by over 95% of biopharmaceutical companies worldwide and have gained extensive recognition from regulatory authorities such as the FDA, NMPA, and EMA.

Beijing XMJ Technology Co., Ltd., as the general agent of Cygnus in China, has established long-term and stable cooperative relationships with numerous well-known domestic pharmaceutical companies and CRO/CMO enterprises. Over the years, XMJ's products and services have helped many enterprises accelerate the R&D phase, improve drug quality, purity and safety, speed up the optimization of R&D processes, shorten product time-to-market, and reduce QC costs. If you are interested in the above-mentioned products, please call XMJ's customer service hotline at 400-050-4006 or visit the official website www.gq44.cn for more information.


 京公網(wǎng)安備 11010802028692號
京公網(wǎng)安備 11010802028692號